In Science, The Little Things Are Usually The Coolest.
It’s currently 11:30 PM, and I’m sitting in a 24-hour coffee shop in downtown Montreal waiting for a train to Toronto that leaves at 6:30 AM. To kill time, I figured that I would write a post about how small things can really get in science by giving everyday analogies for each.
A lot of non-scientific people don’t know this, but a large amount of scientific research is conducted on things that are invisible to the naked eye. For example: proteins, cells, atoms, viruses, DNA, molecules, microbes, etc. can’t be seen without a microscope, x-ray crystallography, or other methods.
In order to get an appreciation for how small or large these can be when compared to each other, I thought it would be cool to start at the smallest and work our way up.
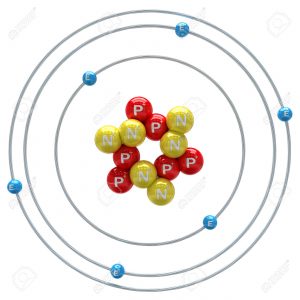
Atoms
Let’s start with an atom. I could go smaller and talk about protons, quarks, and everything else, but I’d rather keep it simple. There’s loads of different atoms, of course. For this example, I’m going to use carbon since it appeals to the organic chemist in me. A carbon atom has an atomic radius of approximately 1.4 x 10^-10 m. Exactly how small is that?
Well, I’m about 1.8 meters tall. That means that I’m approximately 13 billion (12,857,142,857) atoms in height, which is more atoms than the amount of miles it takes to cross from one end of the Solar System to the other.
Proteins
Proteins are the workforce of a cell. They are responsible for nearly everything ranging from sugar metabolism to condensing DNA.
You’d expect something as dynamic and cool as a protein to be pretty large, right? Nope.
On average, a single E. coli bacterium can have up to 10,000,000 proteins in it at any given time, and the average E.coli bacterium is about 0.5 micrometers by 2 micrometers, which is too small for us to see. So, let’s assume that there’s hardly any free space inside this bacterium and that it’s completely crammed to the brim with proteins.
To get an idea of how little space an average protein takes up in an E. coli bacterium, here’s an analogy: the amount of space occupied by a protein inside an E. coli bacterium is roughly equivalent to the space occupied by a standard hot dog stand on the island of Manhattan!
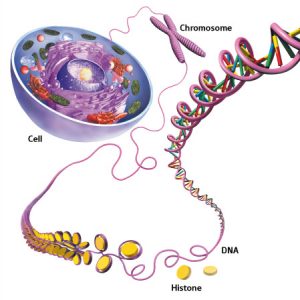
DNA
DNA is something that I first learned about when I watched Jurassic Park at the age of 7.
The average human cell will contain 6 feet of DNA that is condensed into a nucleus that is 10 micrometers in diameter. This is done through wrapping around proteins known as histones. This condensation is equivalent to taking a piece of string that stretches from Victoria to Nanaimo and condensing it until it is only 2 feet long! Insane.
What’s even cooler is that with 37.2 trillion cells in the human body, there’s 42 billion miles of DNA, which is enough to go to Pluto and back 6 times.
Viruses
Viruses are much, much smaller than a human cell. For instance, the influenza virus has a volume of approximately 0.0021 µM^3 whereas an osteoblast has a volume of approximately 4000µM^3. This means that an osteoblast is approximately 2 million times larger than the influenza virus.
Putting this into perspective, an influenza virus landing on an osteoblast (that doesn’t happen, but bear with me) is roughly equivalent to a human (representing the virus) landing on an area of land slightly larger than UVic’s entire campus (representing the cell).
As Yoda would say, ‘size matters not’. This is most definitely true in science: that we’re incapable of seeing are able to carry out countless cool processes, ranging from countless types of body cells all the way to viruses.